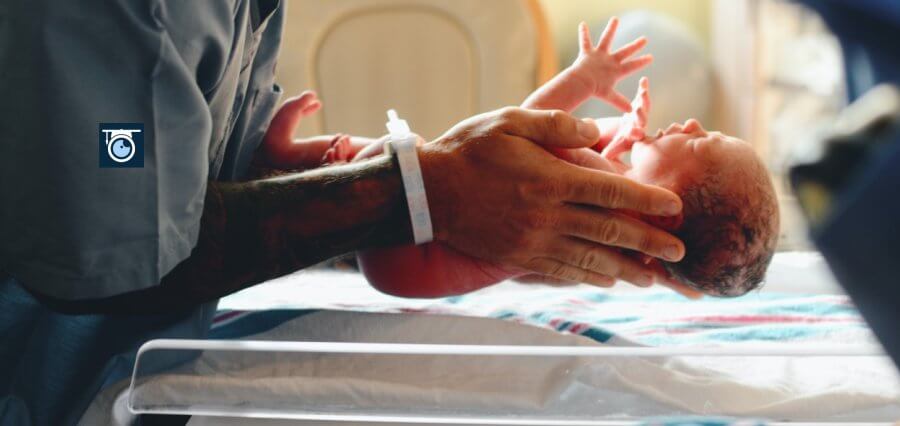
Genomics England will conduct a test to see if scanning newborns’ genomes will hasten the diagnosis of approximately 200 uncommon genetic illnesses and ensure that patients receive treatment more quickly.
Over the following two years, 100,000 newborns’ genomes will be sequenced for the project, which will examine the cost-effectiveness of the strategy and how openly expectant parents are willing to embrace it.
The sequences of newborns will be kept on file even though researchers will only search their genomes for hereditary disorders that manifest in infancy and for which a successful treatment already exists. This could pave the way for future testing to find untreatable adult-onset diseases or other features that are genetically determined.
The fact that infant genomes may follow people from birth to death presents a challenge, according to Sarah Norcross, director of the Progress Educational Trust (PET), a nonprofit organization that enhances options for people with infertility and genetic disorders.
Therefore, protecting this data’s privacy is crucial. According to Norcross, “People must be able to trust that any data collected will only be used in the agreed way, and for the stated purpose.”
Identifying the Disease
In the UK, over 3,000 infants are born every year with an uncommon but treatable disease that could be identified by genome sequencing. Although newborns are presently given a heel-prick test to check their blood for indicators of nine uncommon but catastrophic disorders, like sickle cell disease and cystic fibrosis, whole genome sequencing may make it possible to detect hundreds of additional similar conditions at birth.
Currently, these diseases are frequently not identified until a child exhibits symptoms, frequently following months or years of testing. Biotinidase deficiency, an inherited disorder in which the body is unable to recycle the nutrient biotin, is one such condition. Children who have the condition may endure seizures, developmental delays, and visual or hearing issues, but an early diagnosis and a course of biotin supplements can stop this decline and keep the child healthy.
The chief medical officer of Genomics England, Dr Richard Scott, stated: ”At the moment, the average time to diagnosis in rare disease is about five years. This can be an extraordinary ordeal for families, and it also puts pressure on the health system. The question this programme is responding to is: ‘is there a way that we can get ahead of this?”
Process of Evaluation
Over the next two years, the programme will enrol 100,000 newborns who will voluntarily participate in whole genome sequencing. This will allow researchers to evaluate the technology’s viability and usefulness, as well as its potential to reduce healthcare costs by averting major illnesses.
It will also examine whether a person’s genome might be utilised throughout their lifetime to guide future healthcare decisions and how academics might access an anonymized version of this information to study people as they age. For instance, if a person has cancer later in life, it might be possible to detect and treat them using the genetic information they have stored.
57% of UK citizens support the storing of genetic information in a national database, given that it is only accessible to the sequenced people and the healthcare experts involved in their treatment, according to a study commissioned by PET earlier this year. Only 12% of respondents disagreed.
Scott emphasised that the trial’s goal was to see whether the potential advantages of newborn sequencing stack up and to engage in a sincere national discussion about whether or not people feel comfortable using the technology. The key here is for us to go cautiously and come to an agreement at the national level on what the correct strategy and safeguards are.